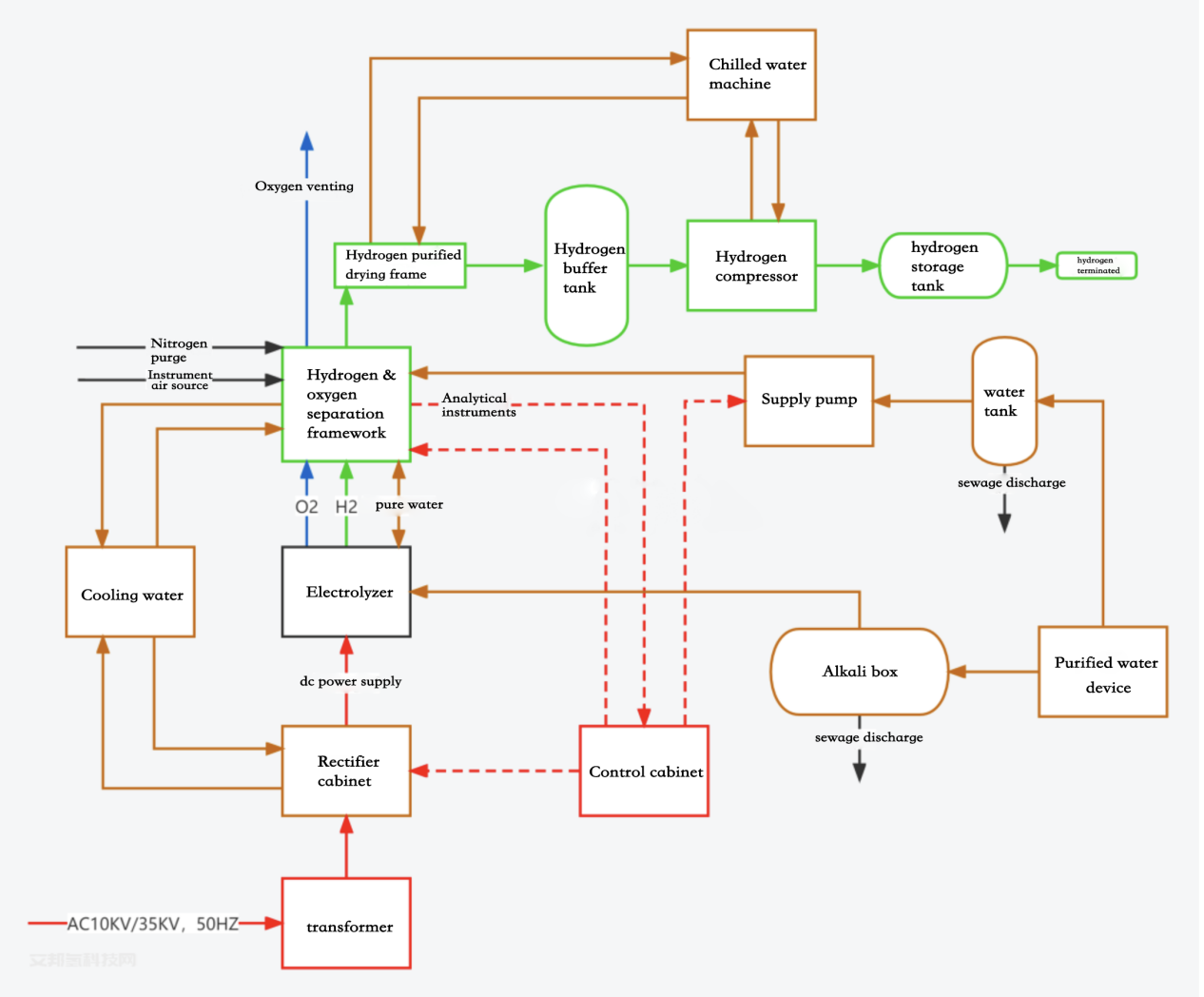
Sashin samar da hydrogen na electrolysis ya haɗa da cikakken saitin kayan aikin samar da ruwa na lantarki. Babban kayan aiki shine:
1. Electrolyzer
2. Gas-ruwa na'urar rabuwa
3. Tsarin bushewa da tsarkakewa
4. Sashin lantarki ya haɗa da: transformer, rectifier cabinet, PLC control cabinet, instrument cabinet, power sharing cabinet, host computer, da dai sauransu.
5. The karin tsarin yafi hada da: alkali tank, albarkatun kasa ruwa tank, ruwa samar famfo, nitrogen kwalban / bas mashaya, da dai sauransu.
6. Gabaɗaya tsarin taimakon kayan aiki ya haɗa da: injin ruwa mai tsabta, hasumiya mai sanyaya ruwa, chiller, compressor iska, da dai sauransu.
A cikin naúrar samar da hydrogen ta electrolytic, ruwa yana bazuwa zuwa wani ɓangare na hydrogen da 1/2 part na oxygen a cikin electrolyzer a ƙarƙashin aikin kai tsaye. Ana aika hydrogen da oxygen da aka samar zuwa mai raba ruwan gas tare da electrolyte don rabuwa. Hydrogen da The oxygen ana sanyaya su ta hanyar na'urorin sanyaya hydrogen da oxygen, kuma mai ɗigo ya kama ya cire ruwa, sannan a aika shi a ƙarƙashin kulawar tsarin sarrafawa; da electrolyte wuce ta cikin hydrogen, oxygen alkali tace, hydrogen, oxygen alkali tace, da dai sauransu karkashin mataki na wurare dabam dabam famfo. ruwa mai sanyaya sa'an nan kuma komawa zuwa electrolyzer don ci gaba da electrolysis.
Ana daidaita matsa lamba na tsarin ta hanyar tsarin kula da matsa lamba da kuma tsarin kula da matsa lamba daban-daban don saduwa da bukatun matakai na gaba da adanawa.
Hydrogen da aka samar ta hanyar lantarki ta ruwa yana da fa'idodin tsafta mai yawa da ƙazanta kaɗan. Yawanci, dattin da ke cikin hydrogen da electrolysis ruwa ke samarwa, iskar oxygen ne kawai da ruwa, kuma babu wasu abubuwa (waɗanda za su iya guje wa guba na wasu abubuwa masu kara kuzari), wanda ke ba da sauƙi don samar da hydrogen mai tsafta. , bayan tsarkakewa, iskar gas da aka samar na iya isa ga alamomin iskar gas na masana'antu.
Hydrogen da na'urar samar da hydrogen ke samarwa yana wucewa ta cikin tanki mai ɗaukar nauyi don daidaita matsa lamba na tsarin da kuma ƙara cire ruwa kyauta a cikin hydrogen.
Bayan hydrogen ya shiga cikin na'urar tsarkakewa hydrogen, hydrogen ɗin da ruwa electrolysis ke samarwa yana ƙara tsarkakewa, kuma ana cire iskar oxygen, ruwa da sauran ƙazanta a cikin hydrogen ta hanyar amfani da ka'idodin catalytic reaction da molecular sieve adsorption.
Kayan aiki na iya saita tsarin daidaitawa ta atomatik don samar da hydrogen bisa ga ainihin halin da ake ciki. Canje-canje a cikin nauyin iskar gas zai haifar da sauye-sauye a cikin matsa lamba na tankin ajiyar hydrogen. Mai watsa matsa lamba da aka sanya a kan tankin ajiya zai fitar da siginar 4-20mA kuma aika shi zuwa PLC kuma Bayan kwatanta ƙimar saiti na asali da yin canjin canji da lissafin PID, ana fitar da siginar 20 ~ 4mA zuwa ma'ajin gyara don daidaita girman girman electrolysis na yanzu, don haka cimma manufar daidaitawa ta atomatik na samar da hydrogen bisa ga canje-canje a cikin nauyin hydrogen.

Kayan aikin samar da ruwa na alkaline electrolysis hydrogen sun hada da tsarin masu zuwa:
(1)Tsarin ruwa danye

Abinda kawai ke amsawa a cikin tsarin samar da ruwa na electrolysis hydrogen shine ruwa (H2O), wanda ke buƙatar ci gaba da cika shi da ɗanyen ruwa ta hanyar famfo mai cike da ruwa. Matsayin sake cika ruwa yana kan mai raba hydrogen ko oxygen. Bugu da ƙari, ƙananan adadin hydrogen da oxygen dole ne a kwashe lokacin barin tsarin. na danshi. Amfanin ruwa na ƙananan kayan aiki shine 1L/Nm³H2, kuma na manyan kayan aiki za'a iya ragewa zuwa 0.9L/Nm³H2. Tsarin yana ci gaba da cika danyen ruwa. Ta hanyar sake cika ruwa, ana iya kiyaye kwanciyar hankali na matakin ruwa na alkali da ƙwayar alkali, kuma za'a iya cika maganin amsawa cikin lokaci. na ruwa don kula da maida hankali na lye.
2)Transformer rectifier tsarin
Wannan tsarin ya ƙunshi na'urori guda biyu: na'ura mai ɗaukar nauyi da na'urar gyarawa. Babban aikinsa shine canza wutar lantarki 10/35KV AC wanda mai gaban-karshen ya bayar zuwa wutar DC da ake buƙata ta hanyar lantarki, da kuma samar da wutar lantarki ga na'urar lantarki. Ana amfani da wani ɓangare na ikon da ake bayarwa don lalata ruwa kai tsaye. Wadannan kwayoyin sune hydrogen da oxygen, ɗayan kuma yana haifar da zafi, wanda mai sanyaya lye ke fitar da shi ta hanyar ruwa mai sanyaya.
Yawancin injinan taranfoma nau'in mai ne. Idan an sanya shi a cikin gida ko a cikin akwati, ana iya amfani da taswirar bushewa. Na'urorin samar da wutar lantarkin da ake amfani da su wajen samar da ruwa hydrogen su ne na'urori na musamman kuma suna buƙatar daidaita su bisa ga bayanan kowane mai amfani da wutar lantarki, don haka kayan aiki ne na musamman.

(3) tsarin rarraba wutar lantarki
An fi amfani da majalisar rarraba wutar lantarki don samar da 400V ko akafi sani da kayan aikin 380V zuwa sassa daban-daban tare da injina a cikin rabuwar hydrogen da oxygen da tsarin tsarkakewa a bayan kayan aikin samar da ruwa na ruwa na lantarki. Kayan aiki sun haɗa da kewayawar alkali a cikin tsarin rabuwa na hydrogen da oxygen. Pumps, famfo masu cika ruwa a cikin tsarin taimako; dumama wayoyi a cikin bushewa da tsarin tsarkakewa, da tsarin taimako da ake buƙata gabaɗayan tsarin, kamar injinan ruwa mai tsabta, chillers, compressors na iska, hasumiya mai sanyaya, da kwampreshin hydrogen na baya-bayan nan, injinan hydrogenation da sauran kayan aikin samar da wutar lantarki kuma ya haɗa da samar da wutar lantarki don hasken wuta, saka idanu da sauran tsarin duk tashar.
(4) tsarin sarrafawa
Tsarin sarrafawa yana aiwatar da sarrafa PLC ta atomatik. PLC gabaɗaya yana amfani da Siemens 1200 ko 1500. An sanye shi da allon taɓawa na hulɗar ɗan adam-kwamfuta, kuma aiki da nunin siga na kowane tsarin kayan aiki da nunin dabaru na sarrafawa ana gane su akan allon taɓawa.
5) Tsarin zagayawa na alkali
Wannan tsarin ya ƙunshi manyan kayan aiki masu zuwa:
Hydrogen da oxygen SEPARATOR - alkali wurare dabam dabam famfo - bawul - Alkali tace - electrolyzer
Babban tsarin shi ne: ruwan alkali da aka haxa shi da hydrogen da oxygen a cikin hydrogen sannan kuma iskar oxygen ke raba shi da mai raba ruwan iskar gas sannan kuma ya koma fanfunan ruwa na alkali. Anan an haɗa mai rarraba hydrogen da mai rarraba iskar oxygen, kuma fam ɗin alkali ruwa zagayawa zai reflux. Ruwan alkali yana kewayawa zuwa bawul da tace ruwa alkali a ƙarshen baya. Bayan tacewa yana tace manyan ƙazanta, ruwan alkali yana yawo zuwa cikin na'urar lantarki.
(6)Tsarin hydrogen
Ana samar da hydrogen daga bangaren lantarki na cathode kuma ya kai ga mai raba tare da tsarin rarraba ruwa na alkali. A cikin na'ura mai rarrabawa, domin ita kanta hydrogen yana da haske, a dabi'ance zai rabu da ruwan alkali kuma ya isa saman sashin mai raba, sannan ya wuce ta cikin bututun don ƙarin rabuwa da sanyaya. Bayan sanyaya ruwa, digo mai kamawa yana kama digo kuma ya kai tsabtar kusan 99%, wanda ya kai ga bushewa da tsarin tsarkakewa na baya.
Ficewa: Ana amfani da ficewar hydrogen don fitarwa yayin farawa da rufewa, aiki mara kyau ko gazawar tsafta, da fitar da kuskure.
(7) Tsarin Oxygen
Hanya don iskar oxygen yayi kama da na hydrogen, amma a cikin wani nau'i na daban.
Ficewa: A halin yanzu, yawancin ayyukan iskar oxygen ana kula da su ta hanyar fitarwa.
Amfani: Ƙimar yin amfani da iskar oxygen yana da ma'ana kawai a cikin ayyuka na musamman, kamar wasu yanayin aikace-aikacen da za su iya amfani da hydrogen da oxygen mai tsabta, kamar masana'antun fiber na gani. Hakanan akwai wasu manyan ayyuka waɗanda suka tanadi sarari don amfani da iskar oxygen. Yanayin aikace-aikacen baya-baya shine samar da iskar oxygen na ruwa bayan bushewa da tsarkakewa, ko amfani da iskar oxygen ta likita ta hanyar watsawa. Koyaya, har yanzu ba a tantance gyare-gyaren waɗannan yanayin amfani ba. Ƙarin tabbaci.
(8)tsarin ruwa mai sanyaya
Tsarin electrolysis na ruwa shine halayen endothermic. Dole ne a samar da tsarin samar da hydrogen tare da makamashin lantarki. Duk da haka, makamashin lantarki da tsarin ruwa na lantarki ke cinyewa ya zarce ka'idar ɗaukar zafi na ruwa electrolysis dauki. Wato wani bangare na wutar lantarki da na’urar lantarki ke amfani da shi ya koma zafi. Wannan bangare Ana amfani da zafi sosai don dumama tsarin zagayawa na alkali a farkon, ta yadda yanayin zafin alkali ya tashi zuwa yanayin zafin jiki na 90± 5 ° C da kayan aiki ke buƙata. Idan na'urar lantarki ta ci gaba da aiki bayan isa ga zafin da aka ƙididdigewa, zafin da aka haifar yana buƙatar amfani da shi Ana fitar da ruwan sanyaya don kula da yanayin yanayin yanayin yanayin electrolysis na yau da kullun. Matsakaicin zafin jiki a yankin amsawar electrolysis na iya rage yawan amfani da makamashi, amma idan yanayin zafi ya yi yawa, membrane na dakin lantarki zai lalace, wanda kuma zai yi illa ga aikin na'urar na dogon lokaci.
Wannan na'urar tana buƙatar kiyaye zafin aiki a ƙasa da 95°C. Bugu da kari, dole ne a sanyaya hydrogen da iskar oxygen da aka samar kuma a rage humided, kuma na'urar gyara siliki mai sanyaya ruwa kuma tana da bututun sanyaya da suka dace.
Jikin famfo na manyan kayan aiki kuma yana buƙatar sa hannun ruwa mai sanyaya.
(9) Nitrogen ciko da tsarin tsabtace nitrogen
Kafin cirewa da aiki da na'urar, dole ne a cika tsarin da nitrogen don gwajin ƙarfin iska. Kafin farawa na al'ada, ana buƙatar lokacin gas na tsarin kuma ana buƙatar tsaftace shi tare da nitrogen don tabbatar da cewa iskar gas a cikin sararin lokaci na iskar gas a bangarorin biyu na hydrogen da oxygen ya nisa daga kewayon flammable da fashewa.
Bayan an rufe kayan aiki, tsarin sarrafawa zai kula da matsa lamba ta atomatik kuma ya riƙe wani adadin hydrogen da oxygen a cikin tsarin. Idan har yanzu ana samun matsa lamba lokacin da aka kunna kayan aiki, babu buƙatar yin tsaftacewa. Koyaya, idan an cire duk matsa lamba, zai buƙaci sake wanke shi. Nitrogen tsaftace aikin.
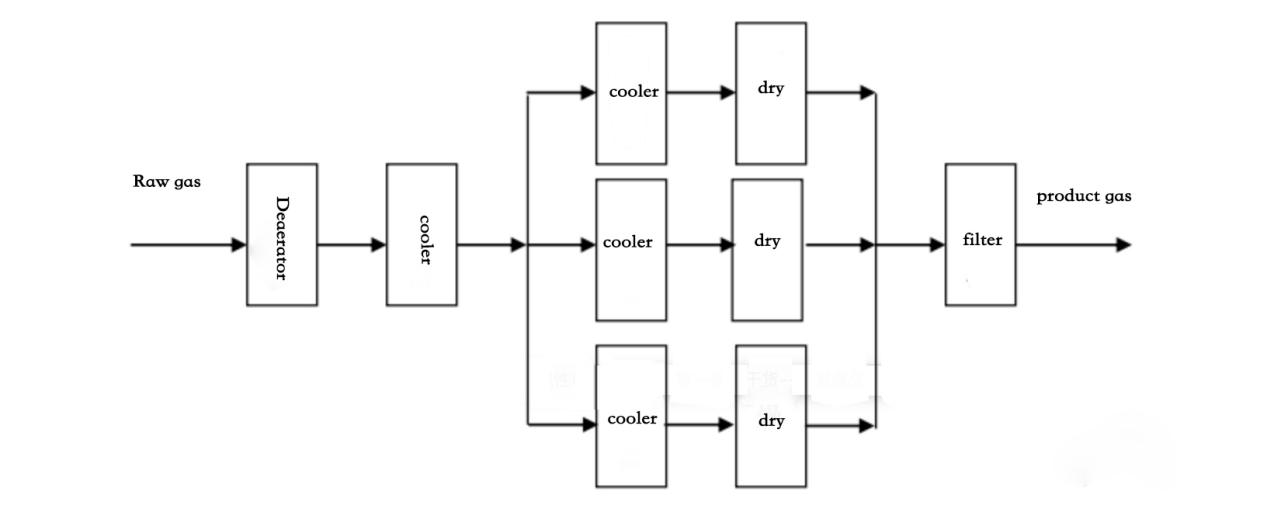
(10) Tsarin bushewar hydrogen (tsarkakewa) (na zaɓi)
Hydrogen da aka samar daga ruwa mai amfani da wutar lantarki ana cire humided ta hanyar bushewa iri ɗaya, sannan kuma a ƙarshe ta zubar da ƙura ta hanyar tace bututun nickel da aka siya don samun busasshen hydrogen. (Bisa ga buƙatun mai amfani don samfurin hydrogen, tsarin zai iya ƙara na'urar tsarkakewa, kuma tsarkakewa yana amfani da palladium-platinum bimetallic catalytic deoxidation).
Ana aika da hydrogen ɗin da na'urar samar da ruwa ta ruwa electrolysis ke aika zuwa na'urar tsarkakewa ta hydrogen ta cikin tanki.
Hydrogen na farko yana wucewa ta hasumiya ta deoxygenation. Karkashin aikin mai kara kuzari, iskar oxygen da ke cikin hydrogen tana amsawa da hydrogen don samar da ruwa.
Tsarin amsawa: 2H2+O2 2H2O.
Bayan haka, hydrogen ɗin ya ratsa ta cikin na'ura mai ba da hanya tsakanin hanyoyin sadarwa (wanda ke sanyaya iskar gas don sanya tururin ruwa a cikin iskar gas don samar da ruwa, kuma ruwan da aka nannade yana fitar da shi kai tsaye daga cikin na'urar ta hanyar tattara ruwa) kuma ya shiga hasumiya ta adsorption.
Lokacin aikawa: Mayu-14-2024




