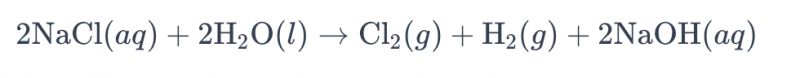Hanyar electrolyzing wani brine bayani ta amfani da titanium electrodes don samar da chlorine yawanci ake magana a kai a matsayin "electrolysis na brine." A cikin wannan tsari, ana amfani da na'urorin lantarki na titanium don sauƙaƙe aikin oxygenation na ions chloride a cikin brine, wanda ke haifar da samar da iskar chlorine. Gabaɗayan lissafin sinadarai don amsawa shine kamar haka:
A cikin wannan ma'auni, ions chloride suna yin iskar oxygenation a cikin anode, wanda ya haifar da samar da iskar chlorine, yayin da kwayoyin ruwa suka ragu a cathode, suna samar da iskar hydrogen. Bugu da ƙari, ions hydroxide suna fuskantar raguwa a cikin anode, suna samar da iskar hydrogen da sodium hydroxide.
Zaɓin na'urorin lantarki na titanium shine saboda kyakkyawan juriya da haɓakar lalata titanium, yana ƙyale shi ya jure yanayin a tsaye yayin electrolysis ba tare da lalata ba. Wannan ya sa wayoyin lantarki na titanium ya zama kyakkyawan zaɓi don electrolysis na brine.
Electrolysis na ruwan gishiri yawanci yana buƙatar tushen wutar lantarki na waje don samar da makamashi don amsawar electrolytic. Wannan tushen wutar lantarki yawanci wutar lantarki ce kai tsaye (DC) saboda halayen electrolytic suna buƙatar daidaitaccen alkiblar gudanawar yanzu, kuma wutar lantarki na DC na iya isar da alkiblar halin yanzu.
A cikin aikin sarrafa ruwan gishiri don samar da iskar chlorine, ana amfani da wutar lantarki mai ƙarancin ƙarfi ta DC. Wutar lantarki na wutar lantarki ya dogara da takamaiman yanayin amsawa da ƙirar kayan aiki, amma gabaɗaya yana tsakanin 2 zuwa 4 volts. Bugu da ƙari, ƙarfin wutar lantarki na yanzu muhimmin ma'auni ne wanda ke buƙatar ƙididdigewa dangane da girman ɗakin amsawa da yawan samarwa da ake so.
A taƙaice, zaɓin samar da wutar lantarki don electrolysis na ruwan saline ya dogara da ƙayyadaddun buƙatun gwaje-gwaje ko hanyoyin masana'antu don tabbatar da ingantaccen amsawa da samun samfuran da ake so.
Lokacin aikawa: Janairu-16-2024